Blog Post: Exploring the potential of non-platinum group metal catalysts
- Time of issue:2022-03-04 23:32
Blog Post: Exploring the potential of non-platinum group metal catalysts
(Summary description)Platinum group metal (PGM) catalysts are great for C-N bond formation but aren’t your only option. What other routes are available with non-PGM catalysts?
Platinum group metal (PGM) catalysts are a staple in any synthetic chemist’s toolbox. Awarded the Nobel Prize in Chemistry in 2010, palladium-catalyzed cross couplings have revolutionized how chemists synthesize molecules – whether for drug discovery, agriculture, or beyond. In fact, when concepting a new synthetic route involving a C-heteroatom bond formation step, many chemists will reach straight for PGM catalysts.
However, there’s a whole world beyond platinum. Copper, nickel, and many more metals are effective tools for cross-couplings in chemical synthesis and should be considered alongside more mainstream methods. Here we examine the advantages and drawbacks of different catalytic approaches in C-N bond formation.
A world of choice
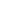
As an example reaction, we focus on the synthesis of N-(biphenyl)-2-aminopyridine. When looking up the product’s retrosynthesis on a chemical database, we found six different routes available, shown below.
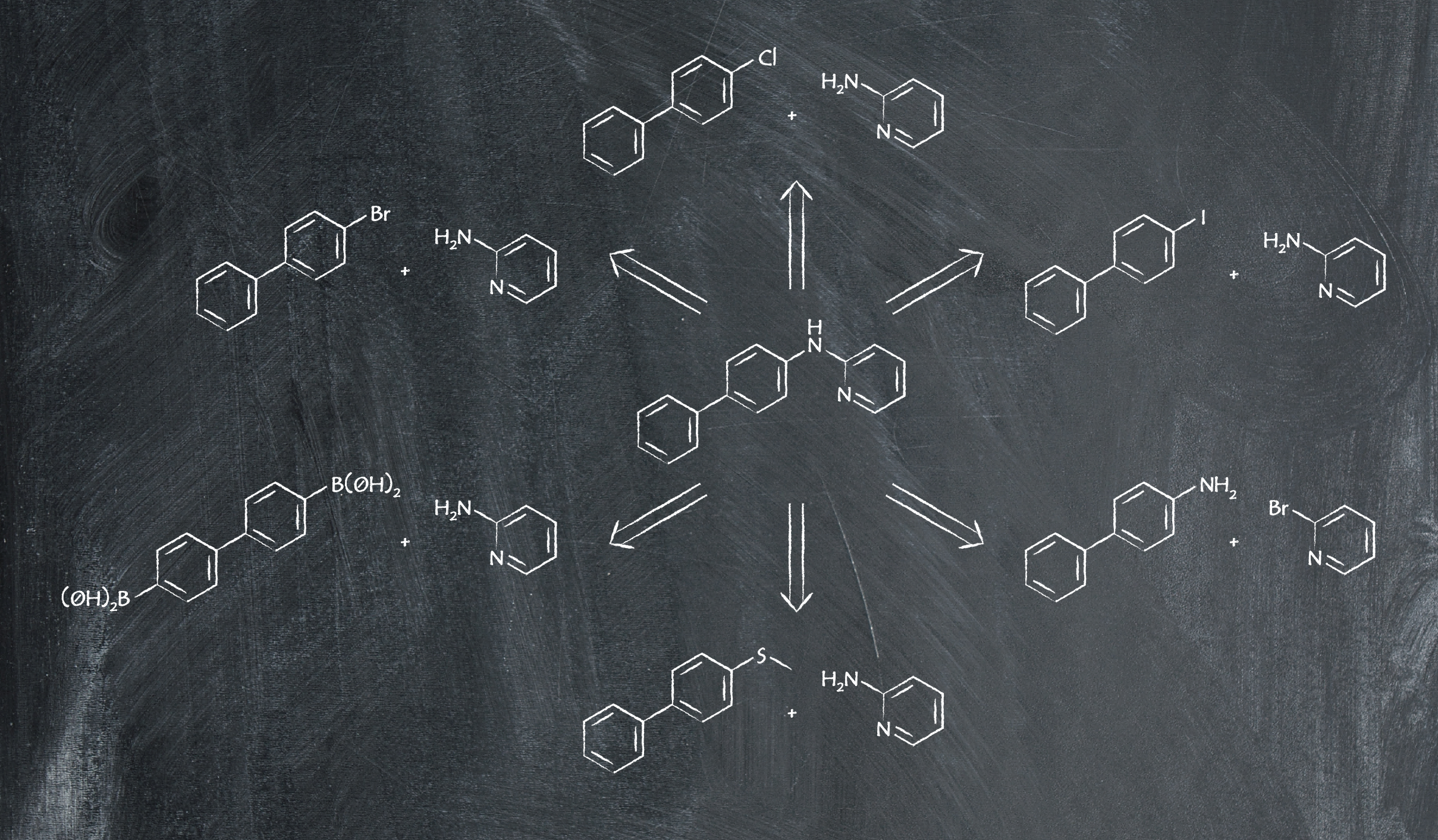
Figure 1: Retrosynthesis of N-(biphenyl)-2-aminopyridine (reported literature routes)
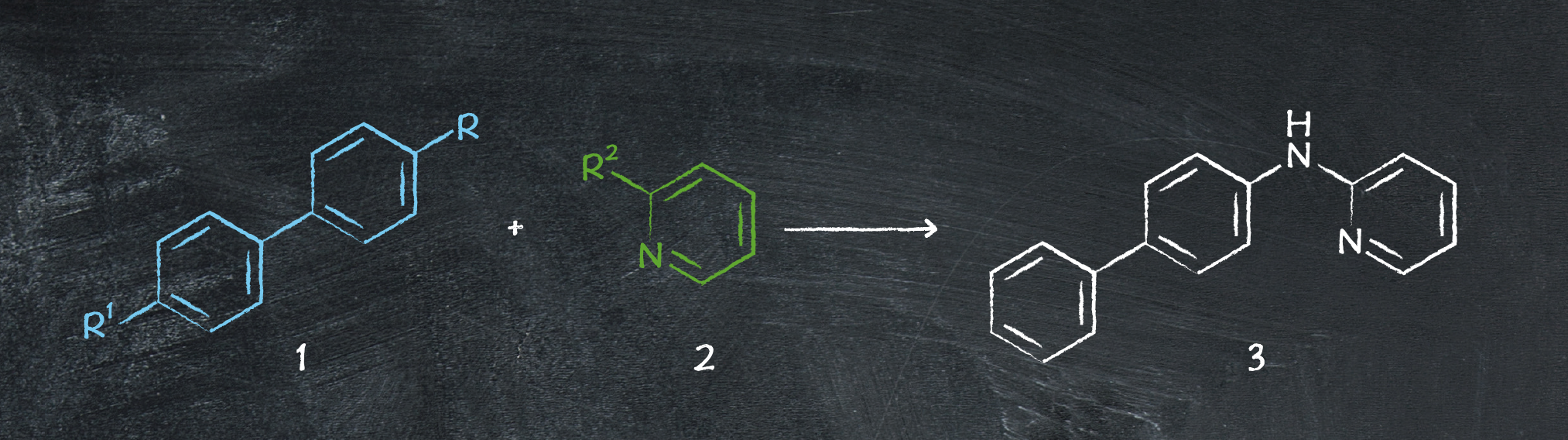
You can see there’s a lot of similarity in the pathways – most of them use 2-aminopyridine as the starting material. However, there are significant differences in the intricacies of the reaction conditions and catalysts used. The six ways to make N-(biphenyl)-2-aminopyridine are shown in the table below, and we examine each reaction in more detail.
Table 1: Comparison of literature reported routes to synthesize 3
Route 1: No catalyst
The first approach is an outlier for using 2-bromopyridine as the electrophilic coupling partner – and it doesn’t use a catalyst, either. Going via an SNAr mechanism instead, the reaction proceeds with a reasonable yield of ~80%, making this a feasible approach. Additionally, the amine starting material is relatively affordable, encouraging its popularity.
From this initial analysis, the method initially seems economically viable. However, the reaction needs temperatures of 160 °C – negating the reagent cost benefits. It’s also limited to materials able to withstand the high temperature without decomposing, so not a versatile option. These issues could, however, potentially be addressed by looking into developing a process in flow rather than batch.
Route 2: Pd-NHC catalysis
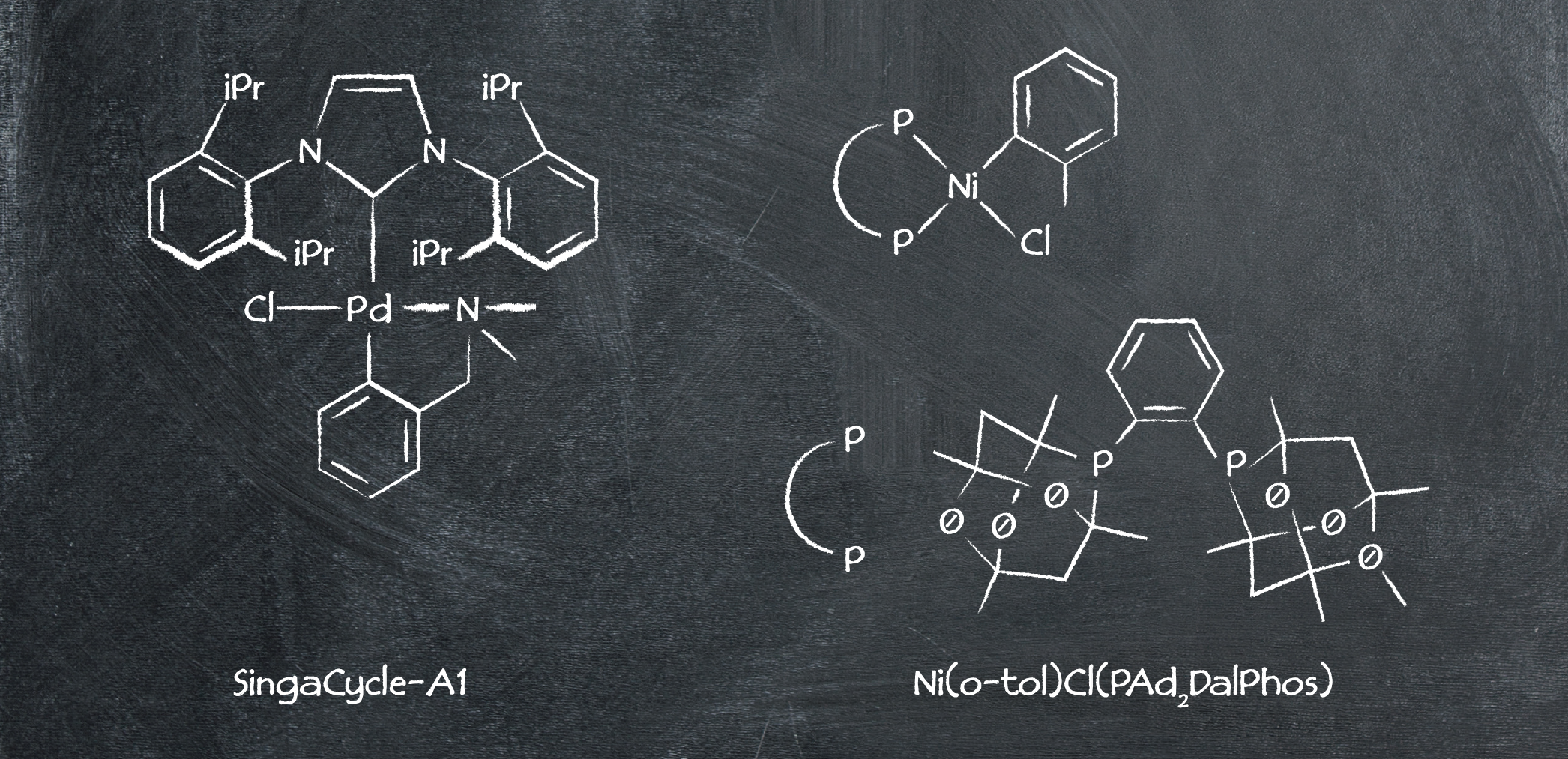
As expected, palladium is a trustworthy catalyst for this reaction, in the form of SingaCycle-A1 (a Pd-NHC catalyst). Giving the highest yield overall of any of the approaches with an impressive 95%, it makes for a promising synthetic route. However, one aspect to factor in is the scarcity of the catalyst – although luckily there are solutions to overcome the catalyst not being commercially available. Additionally, the reaction is driven by a reasonably high temperature of 100 °C – an expensive consideration.
Route 3: Copper-mediated technique
A nice alternative method to palladium is using a copper-mediated Chan-Lam coupling. It’s not clearly catalytic due to the superstoichiometric quantities of Cu(OAc)2.H2O required, but as it’s a relatively cheap starting material the route is promising. Unfortunately, the other materials aren’t quite so affordable, with both the biaryl starting material and the DMSO solvent being costly. In addition, stricter material handling procedures may be needed due to the genotoxic nature of the boronic acid.
The biaryl halide approach
We’ve seen some interesting routes so far, with a variety of starting materials. The following three methods are similar, in that they couple 2-aminopyridine with biaryl halides to obtain the product. However, there’s a range of clever reaction conditions and catalysts to make the target molecule.
Route 4: Copper catalysis
Copper can truly be used as a catalyst in the reaction, in an Ullman coupling. Unlike the previous copper-based method, here only 1% of CuI is needed, demonstrating a favorable approach. Unfortunately, it requires 1,4-dioxane, a possible carcinogen, as the solvent – alternative methods may be preferable to avoid using this when scaling up. Alternatively, it may be necessary to settle for a lower performing alternative solvent.
Route 5: Palladium catalysis
Another palladium-catalyzed approach is available and works nicely with a cost-effective 4-bromobiphenyl starting material. Undergoing a Buchwald-Hartwig amination reaction, the catalytic precursors themselves are less rare than the Pd-NHC reported earlier, simplifying the procedure.
On the surface, this seems like a promising route. However, we were unable to determine the yield of the reaction, or the catalytic loading. If both of these aspects are comparable to previous methods, then the method could be highly successful.
Route 6: Nickel catalysis
The final route available for synthesizing N-(biphenyl)-2-aminopyridine involves a 4-chlorobiphenyl starting material. Although pricier than the bromo-alternative used above, it’s commonly acknowledged that aryl chlorides are cost-effective cross-coupling partners.
The main draw of this method is the non-PGM nickel catalyst – generally cheaper than palladium-based catalysts, it gives a yield comparable to its competitor, making this an appealing option.
The road less traveled
With so many approaches to synthesizing molecules, you don’t have to limit yourself to palladium-catalyzed methods. Often, there are other routes to take which are more economically viable and environmentally friendly. Keeping an open mind and exploring options will maximize the impact of your chemistry.
One of the most promising methodologies here is the nickel-catalyzed route – it’s cost-effective, more environmentally friendly than other options, and uses milder conditions. With many non-PGM catalysts available, there is likely to be an option that suits you and your project’s individual needs.
If you’re considering incorporating non-PGM catalysts into your screening library, Sinocompound can help. Sinocompound can work with you on custom synthesis projects to develop non-commercially available catalysts/ligands, including non-PGM catalysts, for your project needs.
Learn more about Sinocompound’s catalyst products and services to accelerate your project to the next phase: https://en.sinocompound.com/Products_Services.html
1. a) U. Karmakar, D. Das, R. Samanta, Eur. J. of Org. Chem. 2017, 19, 2780-2788. b) Z. Duan, L. Zhang, W. Zhang, L. Lu, L. Zeng, R. Shi, A. Lei, ACS Catal. 2020, 10, 3828-3831.
2. R. Pratap, H. Yorimitsu, Synthesis, 2019, 51, 2705-2712.
3. J. Chen, K. Natte, N.Y.T. Man, S.G. Stewart, X.-F. Wu, Tetrahedron Lett. 2015, 56, 4843-4847.
4. D. Wang, D. Kuang, F. Zhang, Y. Liu, S. Ning, Tetrahedron Lett. 2014, 55, 7121-7123.
5. J.C. Park, S.H. Lee, Y.U. Park, H.S. Ji, M.S. Kang, B.S. Lee, S.P. Hwang, KR2014107026.
6. J. Clark, M. Ferguson, R. McDonald, M. Stradiotto, Angew. Chem. Int. Ed. 2019, 58, 6391-6395.
SINOCOMPOUND © 苏ICP备16062404号-1