Blog Post: Adamantane: a promising motif for ligands in industry?
- Time of issue:2022-02-01 16:06
Blog Post: Adamantane: a promising motif for ligands in industry?
(Summary description)
Organometallic chemistry has been crucial to the evolution of cross-coupling reactions over the past 20 years, largely due to advances in ancillary ligand design. In this four-part blog series, we talk about the rise of bulky adamantyl-containing phosphine ligands and their suitability for industrial applications. In this next blog in our series, we explain why adamantyl-containing ligands show promise in industry.
In our last blog, we highlighted factors that affect which ligands and catalysts are appropriate for scale-up and wider use in industry. We introduced adamantyl-based ligands and their initial promise in catalytic reactions. But what makes these ligands so interesting?
The bulky adamantyl-containing phosphines
Alongside development and supply considerations, the physical structures of catalysts and ligands can influence their chances of commercial success. When considering phosphine ligands, electron-donating substituents on the P atom can encourage a more active catalyst. Additionally, we now know that steric hindrance also plays a huge part in catalytic success.
|
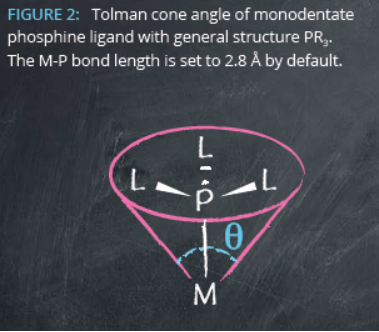
Steric properties are generally quantified by Tolman cone angles (θ). These are defined as the apex angle of a cone emanating from the metal center towards the van der Waals radii of the outermost ligand atoms.
|
In fact, only in 1989 did we understand the link between steric parameterization and catalytic performance – thanks to Osborne and co-workers. The team showed that optimal catalytic activity of Pd-phosphine complexes towards the carbonylation of dichloromethane and chlorobenzene was only observed where the cone angle was ≥160° and the pKa exceeded 6.71.
The adamantane scaffold, with its three fused cyclohexane rings, can offer a great deal of steric bulk. Could this be an effective ligand motif to improve catalytic reactions for scale-up?
Some research has already indicated that this is a promising approach. In 2016, Carrow and co-workers showed that a PAd3-palladacycle could catalyze Suzuki-Miyaura coupling of chloro(hetero) arenes with exceptional TOF and high TON, exceeding other alkylphosphines2.
Adamantane: an improved synthesis
Now we know what makes adamantyl-containing ligands so interesting, we need to consider how accessible these ligands are for scale-up. Adamantane is a natural constituent of fossil fuel with a highly bulky carbon framework. The first synthesis of adamantane was reported in 1941 by Prelog and co-workers3.
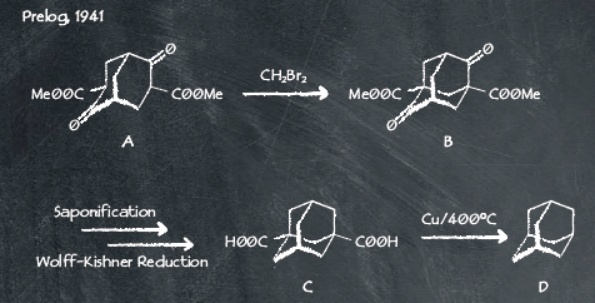
A Wolff-Kishner reduction of the starting material helped secure the desired adamantane product. However, the laborious multi-step method is of limited use practically for application in scale-up due to limited yields and cumbersome steps. While ground-breaking at its time, a much shorter method was developed by Schleyer and co-workers in 19574.
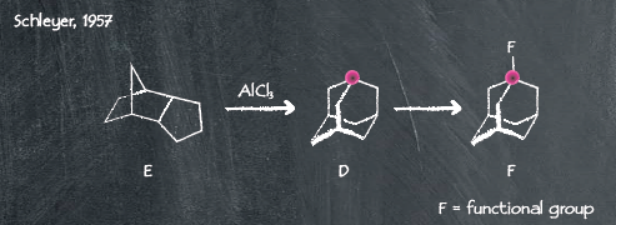
The simplified process reported by Schleyer uses a Lewis acid-catalyzed isomerization of the starting material endotrimethylenenorbornane, giving adamantane in decent yields. The improved reaction procedure has facilitated the use of adamantane as a cheap and widely available starting material. This means researchers have been able to better explore applications of the adamantane scaffold.
It's not just the steric bulk of adamantane that makes this a popular motif, but also the ability to fine-tune the electronic properties. Adamantane can be further functionalised, meaning that many different variations of the scaffold can be investigated and optimised.
How promising are adamantyl-based ligands in industry?
The use of catalytic systems using a Pd-G source and PR3-type phosphine ligands is generally quite well known. In fact, they particularly shine in Suzuki-Miyaura and C-N cross-coupling reactions. However, it’s important to identify optimal ligands, feasible for scale-up, to lower catalyst loading in these reactions. This is why catalyst and ligand screening plays such an important part in exploring chemical space.
The successful use of catalysts featuring the adamantyl moiety has massively increased in literature over the past decade. Beyond C-C bond formation including Suzuki-Miyaura reactions, the ligands have been successful in C-N, C-heteroatom, and even C-halogen bond formation. Although effective for synthesizing many different molecules, importantly these ligands have been demonstrated in the synthesis of pharmaceutically relevant molecules.
All these factors illustrate that adamantyl-based ligands show excellent promise for commercialization, particularly due to their easy scale-up and desirable steric bulk. In our next blog, we’ll cover some of the C-C bond formation reactions from catalysts featuring adamantyl-containing ligands and explore their application in industry.
Download our white paper here to learn more about advanced adamantyl-containing phosphine ligands for challenging cross-coupling reactions.
1 M. Huser, M. T. Youinou, J. A. Osborn, Angew. Chem. Int. Ed. Engl. 1989, 28, 1386-1388.
2 L. Chen, P. Ren, B. P. Carrow, J. Am. Soc. Chem. 2016, 138, 20, 6392-6395.
3 V. Prelog, R. Seiwerth, Ber. Dtsch. Chem. Ges. 1941, 74, 1769-1772.
4 P. von R. Schleyer, J. Am. Chem. Soc. 1957, 79, 3292-3292.
SINOCOMPOUND © 苏ICP备16062404号-1