Blog Post: Catalytic Methods for Atropisomers – with BINAP, H8-BINAP and H8-BINAP-mono oxide in the spotlight
Release date:2024-11-19
Atropisomers are axially chiral isomers that differ in rotation about a molecular axis. These molecules are very common in natural products, but they are difficult to develop due to challenges in obtaining isomerically pure products. Stable axial stereocenters are hard to obtain, and separating the isomers can be very difficult. However, these syntheses are made easier by new catalytic strategies and advances in chromatographic separation, some of which are described in our DrugHunter article.
Four stable atropisomer drugs have been approved by the FDA: vancomycin (1988), colchicine (2009), lesinurad (2015, discontinued), and sotorasib (2021). Several more are currently drug candidates. Drug candidate BMS-986142 is an atropisomer with two axial chiral centers. It is produced using a palladium-catalyzed cross-coupling. An achiral catalyst (Pd(dppf)Cl2) resulted in insufficient stereoselectivity (atropodiastereomeric ratio of 1.4:1), so a screening of chiral ligands was performed. The ligand chosen was (R)-BINAP, which, coupled with Pd(OAc)2, produced the desired product in a 16:1 ratio.
Indeed, the chiral bisphosphine ligand BINAP (2,2’-diphenylphosphino-1,1’-binaphthyl), whose utility earned its developer a Nobel prize, is a key ligand in many asymmetric transformations. While BINAP may often be more effective than other ligands, it is not a golden ticket, and there are many reactions where it does not give the desired results. Frequently, this challenge can be solved with derivatization of the ligand.
H8-BINAP, which has a partially hydrogenated binapthyl moiety, has been found to be a very efficient ligand for many reactions, and frequently superior to other bisphosphine ligands in enantioselective reactions. In a ligand screening for asymmetric Rh(I)-catalyzed cycloaddition, H8-BINAP proved to be the highest yielding ligand, and both BINAP and H8-BINAP outperformed other bisphosphine ligands in catalytic effectiveness. 1Another comparison, in a Rh-catalyzed cyclization of enzymes, showed that H8-BINAP produced the same yield and selectivity as BINAP, but with significantly reduced reaction time (other BINAP derivatives were less effective). 2Experiments in Ir-catalyzed carbonyl reductive coupling for modification of perphenazine showed that H8-BINAP produced significantly better conversion and enantioselectivity compared to other ligands, even BINAP.3 In the synthesis of the drug Lozol, H8-BINAP provided the highest conversion (>95%) and enantioselectivity (91% ee) compared to other bisphosphine ligands.4 One very notable example of this ligand’s use in commercial manufacturing is the production of Ibuprofen. Its synthesis involves an asymmetric hydrogenation catalyzed by Ru(OAc)2 with (S)-H8-BINAP as the ligand.5
Considering an additional structural modification of a bisphosphine ligand, its mono-oxide derivative can turn out to be its catalytically active form, as shown by a paper by Scripps and Bristol-Meyers Squibb on the use of the XantPhos ligand in a palladium-catalyzed arylation reaction.6 Reaction monitoring in this particular case showed that the active mono-oxide catalyst formed rapidly in-situ, which suggests that a pre-formed mono-oxide ligand derivative may be active in related coupling reactions.
Based on this seminal study, there is now an increased interest in trying to understand whether the mono-oxide of other bis-phosphine ligands is the actual active ligand in different transformations. There are few enantioselective methods for the C-H arylation of arenes. H8-BINAP, in company with Pd(dba)2, provides a useful and efficient one for the C-H arylation of arenes to produce atropisomeric (hetero)biaryls.7 The mono-oxide form of the H8-BINAP ligand was used to catalyze highly enantioselective C-H functionalizations, including atroposelective double C-H arylation. This reaction was effective on a wide range of triazoles and pyrazoles, with selectivities up to 97.5:2.5 er and yields frequently greater than 90%.
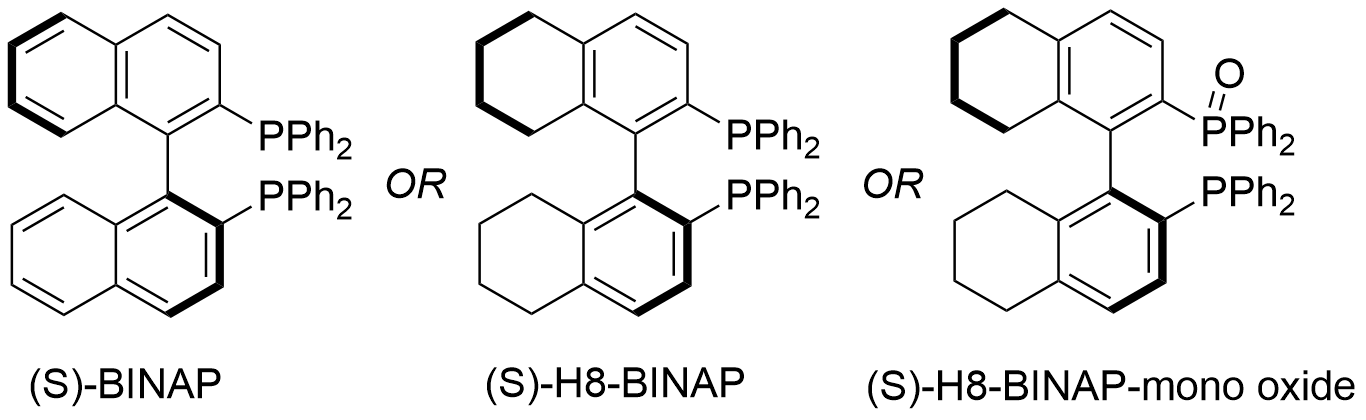
Figure: Structures of BINAP vs H8-BINAP vs H8-BINAP mono-oxide
Choosing the correct catalyst is critical for selectivity in reactions that produce axially chiral molecules. But access to ideal catalysts does not need to be a barrier to ideal reactions, even with tricky products like atropisomers. Sinocompound provides commercial scale quantities of BINAP, H8-BINAP, and other critical ligands and catalysts. We are experts in the use, manufacture, and optimization of homogenous catalysts for pharmaceutical and fine chemical synthesis, including synthesis of not yet commercially available catalysts and ligands, as well as catalyst screening.
1 M. Lin, G. Y. Kang, Y. A. Guo, Z. X. Yu, J. Am. Chem. Soc. 2012, 134, 398-405.
2 Y. Oonishi, S. Masusaki, S. Sakamoto, and Y. Sato, Angew. Chem. Int. Ed. 2019, 58, 8736-8739.
3 K. Spielmann, M. Xiang, L. A. Schwartz, and M. J. Krische, J. Am. Chem. Soc. 2019, 141, 14136-14141.
4 C. B. Yu, X. Li, and Y. G. Zhou, Asian J. Org. Chem. 2019, 8, 1118-1121.
5 T. Uemura, X. Zhang, K. Matsumura, N. Sayo, H. Kumobayashi, T. Ohta, K. Nozaki, H. Takaya, J. Org. Chem. 1996, 61, 5510-5516.
6 Ji, Y.; Plata, E.; Regens, C. S.; Hay, M.; Schmidt, M.; Razler, T.; Qiu, Y.; Geng, P.; Hsiao, Y.; Rosner, T.; Eastgate, M. D.; Blackmond, D. G. J. Am. Chem. Soc. 2015, 137 (41), 13272–13281.
7 Nguyen, Q.-H.; Guo, S.-M.; Royal, T.; Baudoin, O.; Cramer, N. J. Am. Chem. Soc. 2020, 142 (5), 2161–2167.