Blog Post: Palladium precatalysts for efficient reactions
Release date:2024-05-31
Palladium catalysis allows for a wide range of bond-forming transformations and has a long history of use across organic synthesis industries. These catalysts are often created in situ from a Pd precursor and a free ligand. However, this separate catalyst-forming step can lead to undesired side reactions or even catalyst suppression. With preformed catalysts (precatalysts), the ligand is already associated with the metal center, and therefore facilitates formation of the catalytically active LPd(0) species.
The Buchwald Pd G6 precatalysts1,2 are an excellent example of a catalyst family that can open doors to otherwise challenging reactions. These Pd(II) compounds are oxidative addition complexes3 which are effective for C-N, C-O, and C-F cross-coupling reactions, without producing potentially inhibitory byproducts. They are highly efficient, easily handled, and are stable enough to allow for easy long-term storage.
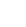
Chemists at Pfizer used a G6 precatalyst to their advantage during pilot plant scale development of an API (PF-07059013) aimed to treat sickle cell disease.4 The initial Buchwald-Hartwig C-O coupling was catalyzed by Pd2dba3-tBuXPhos, which was formed in-situ, resulting in precipitation of Pd black. This was attributed to the bulky ligand not efficiently
complexing to the Pd(0) precatalyst. An initial attempt at replacing the catalyst with tBuXPhos Pd G3 gave a faster reaction and no precipitation, but resulted in impurity growth from unwanted side coupling reactions. Their solution was to switch to the precomplexed catalyst tBuBrettPhos Pd G6 bromide, synthesized separately and used as a crude solution in the coupling reaction. As a result, they achieved full reaction conversion at a high reaction rate and low catalyst loading (0.1 mol%). No Pd black was produced, which simplified purification and reduced the time in plant.
A recent paper by Prof. Buchwald described a new palladium ligand called GPhos, which allows for C-N coupling reactions to be run at room temperature, and can work effectively with sterically hindered substrates such as aryl halides and amines.5 The group made structural adjustments to the dialkylbiaryl monophosphine framework of the ligand BrettPhos for a more effective design. They added a bulky Ot-Bu group for stability, removed the i-Pr group to reduce overall bulk, and added an OMe group for a faster reaction rate than the BrettPhos ligand. The GPhos ligand is effective both at room temperature and at 90ºC, where the reaction rates increase further. It allows for C-N cross-coupling between complex substrates: primary amines (including unhindered, five-membered-ring N-heterocycle-containing, and α-tertiary primary amine nucleophiles) and aryl halides. The favorable combination of applying the GPhos ligand in the form of its Pd G6 precatalyst could provide advantages in a chemical process.

G6 precatalysts are not only available with Buchwald-type dialkylbiaryl phosphine ligands, but other aryl, halide, amine, and phosphine-based ligands as well. Pd(PAd3)(p-FC6H4)Br is an on-cycle precatalyst that has been applied in Suzuki-Miyaura cross-coupling using fluorinated arylboronic acids.6 The on-cycle, air-stable [(Np3P)Pd(Ar)Br]2 and (Np3P)Pd(Ar)(amine)Br precatalysts have been demonstrated in N-arylation reactions between aryl bromides and aniline derivatives.7 Finally, (tBu3P)Pd(Ar)X precatalysts are reported to work effectively in Suzuki-Miyaura couplings of sensitive boron substrates, as well as sterically hindered substrates.8
Precatalysts can enhance reaction performance by reducing the number of steps required to form the active catalyst and therefore reduce the chance for impurity formation. As a result, reaction rate is increased, purification requirements are reduced, and catalyst loading can also be decreased.
Catalyst diversification increases the likelihood of finding the perfect catalyst for a troublesome reaction, and the use of advanced palladium precatalysts can solve synthetic problems across the industry. Sinocompound can supply a wide range of Pd precatalysts, including ones with extremely bulky phosphine ligands that otherwise cannot be readily formed. From gram to kilogram quantities, Sinocompound can help to enhance catalyst libraries, support reaction development, and provide efficient large-scale manufacturing.
1 B.T. Ingoglia, S.L. Buchwald, Org. Lett. 2017, 19, 2853.
2 R.P. King, S.W. Krska, S.L. Buchwald, Org. Lett. 2021, 23, 6030.
3 A.G. Sergeev, A. Zapf, A. Spannenberg, M. Beller., Organometallics 2008, 27, 297.
4 C. Abercrombie et. al., Org. Process Res. Dev. 2023, 27, 866.
5 S.D. McCann, E.C. Reichert, P.L. Arrechea, S.L. Buchwald, J. Am. Chem. Soc. 2020, 142, 15027.
6 L. Chen, H. Francis, B.P. Carrow, ACS Catal. 2018, 8, 2989.
7 H. Hu, C.E. Burlas, S.J. Curley, T. Gruchala, F. Qu, K.H. Shaughnessy, Organometallics, 2020, 39, 3618.
8 Y.N. Timsina, G. Xu, T.J. Colacot, ACS Catal. 2023, 13, 8106.