Blog Post: New ligands enhance copper catalysis
Release date:2023-12-08
C-heteroatom bond formation is an essential part of organic synthesis and is critical for the preparation of substituted aromatic groups present in pharmaceuticals. Palladium has commonly been used to catalyze these reactions, because palladium-catalyzed reactions have been extensively studied and are well understood. However, palladium and other platinum-group metals (PGM) have drawbacks like other precious metal catalysts, such as limited natural occurrence, higher prices, and frequent price/availability fluctuations.
Copper catalysis provides a viable alternative to PGM catalysis. Copper is a more abundant and less expensive metal, and can result in milder reaction conditions and less air-sensitive reactions. Copper catalysts have different reactivities than PGM catalysts, and can provide solutions for challenging synthetic routes. Newly developed copper ligands like oxalic diamides, picolinamides, pyrrole-ol, and diamines have opened up a wide range of possibilities for cross-coupling reactions.
Variously substituted oxalic diamides1 can provide Cu ligands for hydroxylation2 and alkoxylation of aryl halides.3 Some of these allow for low catalyst loading and still give good yield, such as BTMPO in the Cu-catalyzed C-N coupling reaction of amines with (hetero)aryl chlorides. Other oxalic diamide ligands can be used for C-N, C-O, C-S and C-C bond formations.4,5
Chemists at Pfizer found that picolinamide ligands, such as 6-hydroxypicolinamide, can be effective for Cu-catalyzed coupling of heteroaryl primary amines and (hetero)aryl bromides. With increased catalyst loading and reaction temperature, aryl chlorides undergo the reaction as well.6
Pyrrole-ol has been found to be a useful ligand to catalyze C-N couplings with sterically hindered substrates. Optimization by chemists at Abbvie showed that CuI and a benzyl-substituted pyrrole-ol ligand can enable the coupling of sterically hindered amines, anilines, and amides with ortho-substituted aryl iodides.7,8
The cyclic diamine ligand trans-CyDMEDA is well-established in copper-catalyzed reactions, including in amidation of aryl halides and N-arylation of heterocycles.9 It's been used to synthesize pharmaceuticals by GSK, BMS, Merck, and AstraZeneca. However, for transportation purposes it is classified as dangerous goods, which presents logistics challenges. Its MSA salt, on the other hand, is an air-stable solid and shows similar reactivity in coupling reactions, and can potentially be a safer alternative ligand without sacrificing reaction efficiency. 10
The Ullmann reaction is a particular type of Cu-catalyzed coupling, traditionally a nucleophilic aromatic substitution with an aryl halide. Kim et. al. developed anionic ligands for Cu-catalyzed amination of aryl bromides.11 Density functional theory (DFT) calculations were used to design ligands that increased electron density on Cu and stabilized the anionic Cu complex with a π-interaction. A series of N1,N2-diarylbenzene-1,2-diamine ligands were designed and utilized in coupling reactions with a wide variety of substrates. The ligand L4 allows for the use of larger alkoxide bases for the coupling of methoxide-sensitive substrates in good yield. The ligand L5 allows for the coupling of more hindered substrates.
Cu(MeCN)4PF6 catalyzes the coupling of amines with aryl boronic acids without the denucleation problems that can occur with Cu(OAc)2 and other Cu salts. It's been used for Chan-Evans-Lam coupling reactions by both GSK12 and Merck13, and is available in commercial quantities from Sinocompound.
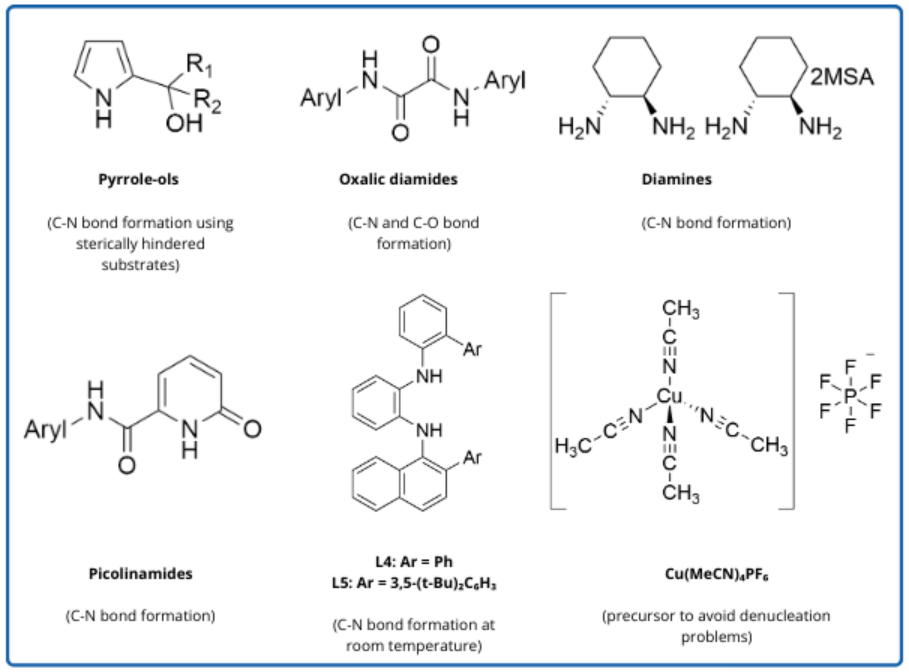
Figure 1. Prominent ligands and Cu precursor for copper-catalysis. Available in g and kg quantities from Sinocompound (MSA = methane sulfonic acid).
All of these ligands expand the library of useful coupling reactions, giving chemists more options during catalyst screening and high-throughput experimentation.
Exploring non-precious metal catalysis could reduce production cost of an API, especially if included in the initial stages of route-scouting. New developments in ligands and mechanisms of catalysis have expanded the substrate scope of copper-catalyzed reactions. Cu catalyst precursors such as CuI are generally readily available.
Sinocompound can provide these and a wide range of copper ligands for all of your process needs, from development scale to commercial quantities for manufacturing. Together, we can explore catalytic processes that are safer, milder, and less costly than traditional PGM catalysis.
1 W. Zhou, M. Fan, J. Yin, Y. Jiang, D. Ma, J. Am. Chem. Soc. 2015, 137, 11942-11945.
2 S. Xia, L. Gan, K. Wang, Z. Li, and D. Ma, J. Am. Chem. Soc. 2016, 138, 13493-13496.
3 J. R. Wang, Z. Q. Song, C. Li, and D. H. Wang, Org. Lett. 2021, 23, 8450-8454.
4 Z. Chen, Y. Jiang, L. Zhang, Y. Guo, and D. Ma, J. Am. Chem. Soc. 2019, 141, 3541-3549.
5 Q. Yang, Y. Zhao, D. Ma, Org. Process. Res. Dev. 2022, 26, 1690-1750.
6 D. J. Bernhardson, D. W. Widlicka, and R. A. Singer, Org. Process Res. Dev. 2019, 23, 1538- 1551.
7 A. Modak, A. J. Nett, E. C. Swift, M. C. Haibach, V. S. Chan, T. S. Franczyk, S. Shekhar, and S. P. Cook, ACS Catal. 2020, 10, 10495-10499.
8 A. de Gombert, A. Darù,.Tonia S. Ahmed, M. C. Haibach, R. L. Matsuura, C. Yang, R. F. Henry, S. P. Cook, S. Shekhar, and D. G. Blackmond, ACS Catal. 2023, 13, 2904-2915.
9 J. C. Antilla, J. M. Baskin, T. E. Barder, and S. L. Buchwald, J. Org. Chem. 2004, 69, 5578-5587.
10 P. E. Maligres, S. W. Krska, and P. G. Dormer, J. Org. Chem. 2012, 77, 7646-7651.
11 S.-T. Kim, M. J. Strauss, A. Cabré, S. L. Buchwald, J. Am. Chem. Soc. 2023, 145, 6966-6975.
12 J.C. Vantourout, L. Li, E. Bendito-Moll, S. Chabbra, K. Arrington, B.E. Bode, A. Isidro-Llobet, J.A. Kowalski, M.G. Nilson, K.M.P. Wheelhouse, J.L. Woodard, S. Xie, D.C. Leitch, A.J.B. Watson, ACS Catal. 2018, 8, 9560.
13 Z.J. Song, P. Maligres, C. Molinaro, G. Humphrey, J. Fritzen, J. Wilson, Y. Chen, Org. Process Res. Dev. 2019, 23, 2354.