Nickel catalysts: modular, inexpensive, and robust
Release date:2023-09-18
Transition metal catalysts are an essential part of every organic chemist’s toolbox. For cross-coupling, that transition metal has traditionally been palladium.
Palladium catalysts are easy to use and manipulate and are well-studied. However, palladium and other platinum-group metals (PGMs) are expensive and have high environmental costs. The exploration of non-PGM catalysis, which are often less costly in both regards, has opened up new opportunities in reactivity and route optimization.
Exploration of diverse catalytic routes to a particular molecule shows that non-PGM based catalysts can be more cost-effective than PGM catalysts, with comparable yields. Non-PGM catalysts can also be more environmentally friendly or allow for milder conditions, while providing critical enantioselectivity.
Nickel-based catalysts in particular have shown potential for use in cross-coupling reactions. Ligands traditionally used with PGM catalysts can also work with nickel in similar bond-forming reactions.
Figure 1 highlights selected nickel catalysts and ligands that have been recently described in the literature.
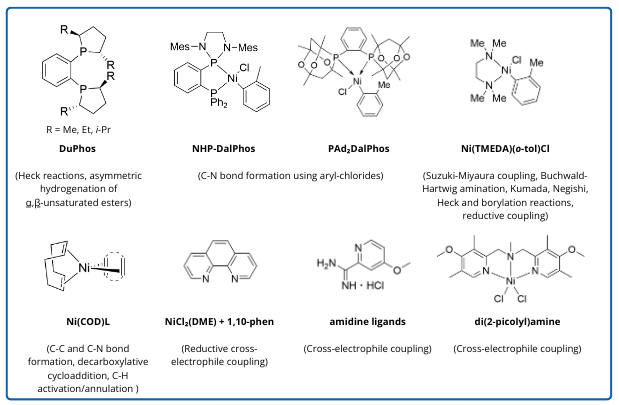
Figure 1. Prominent nickel catalysts and ligands.
Ni catalysts with DuPhos ligands have been used for asymmetric hydrogenation of α,β-unsaturated esters.1 These reactions use relatively mild conditions, tolerate a range of substrates, and result in good yields and good enantiomeric selectivity. In another example, excellent enantioselectivity was achieved in a Ni-catalyzed hydrogenation of 2-oxazolones using the related ligand Ph-BPE.2
New catalytic metals lead to new ligand exploration. While Ni-catalyst development often starts with PGM ligands, other ligands are specifically designed for use with Ni. Two of these ligands are NHP-DalPhos3 and PAd2DalPhos.
One route to synthesize N-(biphenyl)-2-aminopyridine uses Ni(o-tol)Cl(PAd2DalPhos) to catalyze a C-N bond formation. Comparisons to other routes show that the Ni-catalyzed route has mid-range conditions and a good comparative yield (89%). 4
Phosphine ligands are common in PGM catalysts, but N- and O-based ligands perform better in Ni-catalyzed cross-electrophile coupling. Hansen, Weix and colleagues screened the Pfizer chemical library and found that bipyridine (dmbpy and derivatives) and carboxamidine ligands gave the best performance in Ni-catalyzed cross-electrophile couplings. They eventually created a third-generation ligand library of pyridyl-2-carboxamidine derivatives, which gave greater yields and less byproducts than the control group of bipyridine ligands.5
Rago and colleagues at Abbvie developed novel di(2-picolyl)amine (DPA) ligands in single-step syntheses and applied them to Ni-catalyzed cross-electrophile couplings. Modular, inexpensive, and robust, these ligands coordinated with nickel to create C-C bonds with up to 63% yield and with reactivity comparable to the dtbbpy ligand. 6
Tran, Engle and collaborators at Bristol Myers Squibb developed ten bench-stable nickel-olefin complexes for use as pre-catalysts, taking inspiration from the common Ni(0) ligand 1,5-cyclooctadiene (COD). They began with Ni(COD)(DQ), an air-stable 18-electron complex, and proceeded to develop further variations on the Ni(COD)(L) formula. These complexes, involving ligands from the quinone, cyclopentadienone, thiophene-S-oxide, and fulvene families, were applied to C-C and C-N bond-forming reactions, often with improved stability ,and reactivity over the initial duroquinone-based complex.7,8
Ni(TMEDA)(o-tolyl)Cl is an air-stable precursor that kicked off more reports of Ni-based methodology. It has been demonstrated to be effective in a wide range of reactions, including Suzuki-Miyaura coupling; Buchwald-Hartwig amination; Kumada, Negishi, Heck and borylation reactions; and reductive coupling. 9
Few nickel-catalyzed transformations have been reported in large-scale syntheses. One of these is a key pharmaceutical intermediate for a Bristol Myers Squibb candidate. The nickel catalyst, formed in-situ from NiCl2(DME) and 1,10-Phen, was used to produce the product on kg scale.10
Nickel catalysis, while still in early stages of its development, holds significant promise for efficient, inexpensive cross-coupling reactions.
Industrial researchers examining catalytic processes to make their products may discard promising novel routes due to concerns about sourcing the required catalysts or ligands in sufficient quantity. This includes new non-PGM technology, which is still in its infancy. Sinocompound wants to remove that hurdle and encourage industrial R&D groups to explore recently developed catalytic systems. Together, we can work towards chemical practices that are more efficient, more inexpensive, and more sustainable.
1 M. Shevlin, M.R. Friedfeld, H. Sheng, N.A. Pierson, J.M. Hoyt, L.-C. Campeau, P.J. Chirik, J. Am. Chem. Soc. 2016, 138, 3562-3569.
2 Y. Liu, Z. Yi, X. Yang, H. Wang, C. Yin, M. Wang, X.-Q. Dong, X. Zhang, ACS Catal. 2020, 10, 11153-11161.
3 A. V. Gatien, C. M. Lavoie, R. N. Bennett, M. J. Ferguson, R. McDonald, E. R. Johnson, A. W. H. Speed, and M. Stradiotto, ACS Catal. 2018, 8, 5328-5339.
4 J. Clark, M. Ferguson, R. McDonald, M. Stradiotto, Angew. Chem. Int. Ed. 2019, 58, 6391-6395.
5 E. C. Hansen, D. J. Pedro, A. C. Wotal, N. J. Gower, J. D. Nelson, S. Caron, and D. J. Weix, Nat. Chem., 2016, 8, 1126-1130.
6 A. J. Rago, A. Vasilopoulos, A. W. Dombrowski, and Y. Wang, Org. Lett., 2022, 24, 8487-8492.
7 V. T. Tran et. al., Angew. Chem. Int. Ed. 2023, 62, e202211794.
8 V. T. Tran, Z. Q. Li, O. Apolinar, J. Derosa, M. V. Joannou, S. R. Wisniewski, M. D. Eastgate, and K. M. Engle, Angew. Chem. Int. Ed. 2020, 59, 7409-7413.
9 a) J. D. Shields, E. E. Gray, and A. G. Doyle, Org. Lett. 2015, 17, 2166-2169; b) J. Magano and S. Monfette, ACS Catal. 2015, 5, 3120-3123; c) D. K. Kölmel, J. Meng, M. H. Tsai, J. Que, R. P. Loach, T. Knauber, J. Wan, and M. E. Flanagan, ACS Comb. Sci. 2019, 21, 588-597.
10 S. K. Nimmagadda, S. Korapati, D. Dasgupta, N. A. Malik, A. Vinodini, A. S. Gangu, S. Kalidindi, P. Maity, S. S. Bondigela, A. Venu, W. P. Gallagher, S. Aytar, F. G. Bobes, and R. Vaidyanathan, Org. Process Res. Dev. 2020, 24, 1141-1148.