Blog Post: The potential of base metals for asymmetric transformations
Release date:2022-12-19
When determining the ideal methodology for a particular catalyzed reaction, many factors must be considered. Good yield is a high priority, but a chemist will also consider the cost and availability of the catalyst, the reaction conditions, and whether the reagents/solvents are environmentally friendly. Less common reaction conditions or catalysts may end up providing optimal results.
Consider the 2,5-disubstituted bisphospholane ligands DuPhos and BPE, developed by Burk and co-workers in the early 1990’s.1,2 Originally pioneered in highly efficient enantioselective hydrogenation of C=C and C=N double bonds, these ligands have been shown to be effective in other metal-catalyzed reactions as well.
Asymmetric transformations have often used DuPhos and BPE ligands, usually ligated to rhodium complexes (a platinum-group metal (PGM)). These ligands allow the catalysis of hydrogenation, hydroformylation, hydrosilylation, and hydroacylation with robustness, high activity, and excellent enantioselectivity. Enantiomeric purity of the product can be greater than 99.5% ee.
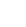
Chemists at Dow developed an extremely efficient asymmetric hydrogenation route for the gram-scale synthesis of enantiomerically enriched succinamic acid derivatives, using a rhodium-DuPhos catalyst system (Scheme 1).3
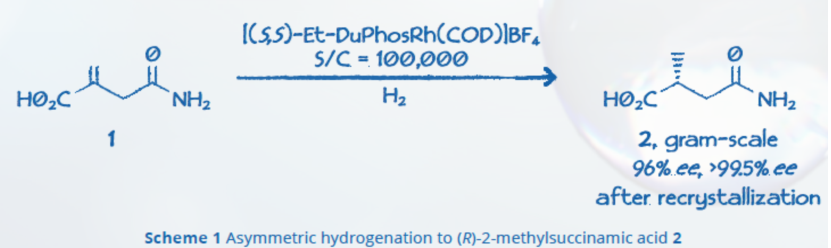
However, as we mentioned in a previous blog post, the use of base metals in these reactions can be much more cost-effective than that of PGM catalysts, without sacrificing either yield or enantioselectivity. For example, the Me-DuPhos ligand was shown to be an effective catalyst in conjunction with non-PGM metals such as nickel.
Chirik and co-workers developed a highly active and enantioselective phosphine nickel catalyst for the asymmetric hydrogenation of α,β-unsaturated esters, using the (S,S)-Me-DuPhos ligand.4 This reaction uses an air-stable nickel source under relatively mild conditions in methanol, and tolerates a range of substrates with electron donating and withdrawing functional groups, with products up to 99% isolated yield and 96% ee (Scheme 2).
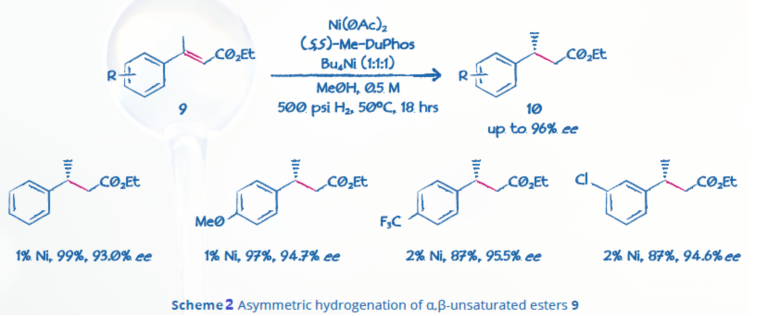
Another Ni-catalyzed asymmetric hydrogenation of α,β-unsaturated esters used DMF as the hydride source and (R,R)-Me-DuPhos as the ligand, and produced saturated esters in good yield with up to 93% ee (Scheme 3).5
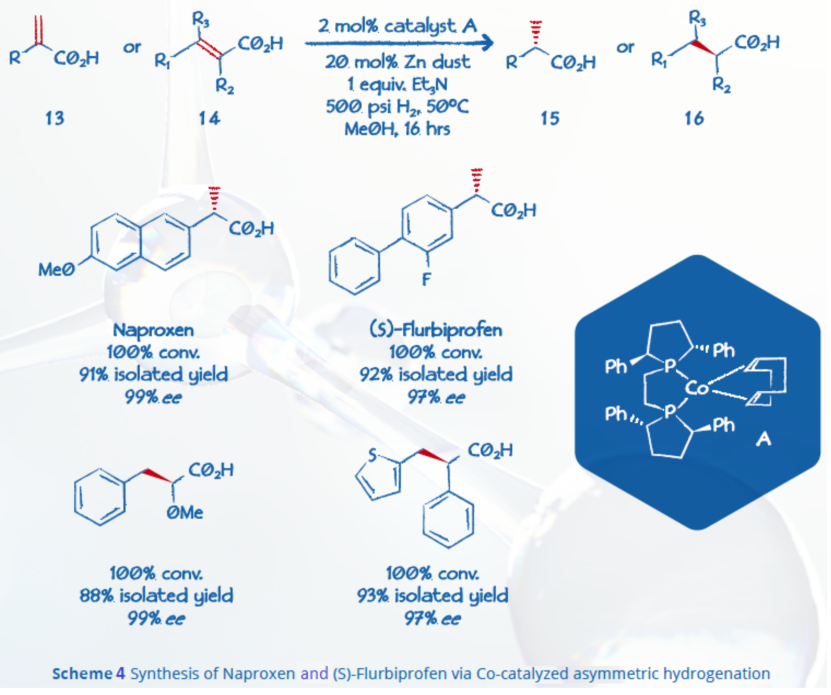
BPE coupled with cobalt or copper has been shown to be effective in several reactions. Chirik and co-workers also reported an asymmetric hydrogenation of α,β-unsaturated carboxylic acids with a cobalt-BPE catalytic system (Scheme 4).6 The methodology, which also includes zinc, produces chiral carboxylic acids such as Naproxen with high yields and enantioselectivities (up to 99% ee).
Zhang and co-workers recently took advantage of a cobalt catalyst bearing electron-donating ligand Ph-BPE series to produce the gram-scale syntheses of key intermediates (Scheme 5) for chiral drugs Sacubitril and Artemisinin with high activity and enantioselectivity (>99% ee).7

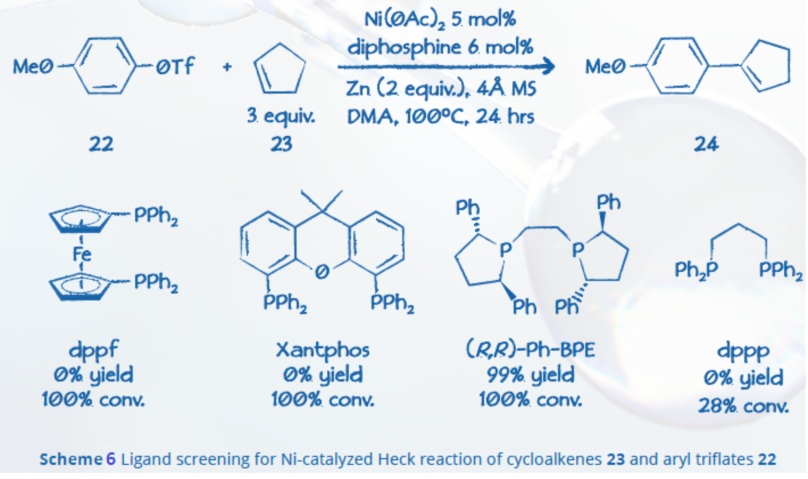
Chi et al. reported a recent Ni-catalyzed Heck reaction between aryl electrophiles and cycloalkenes, using Ni(0) catalysts ligated by chelating (R,R)-Ph-BPE (Scheme 6). The BPE ligand allowed 99% yield and 100% conversion.8
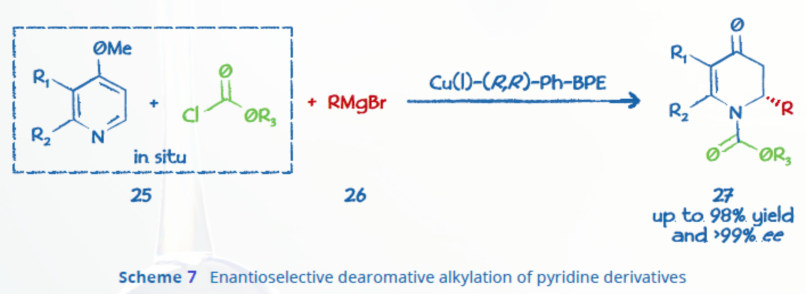
A general copper-BPE catalytic methodology for the enantioselective dearomative alkylation of pyridine derivatives was described by Harutyunyan and co-workers (Scheme 7).9 The methodology produced nearly enantiopure (>99% ee) chiral dihydro-4-pyridones with very high yields (98%).
DuPhos and BPE ligands have mostly been used in academic settings, with few industrial applications reported. This may be due to a previous lack of commercial availability of these ligands on a sufficient scale. With their robustness, high activity and excellent enantioselectivity, they are promising candidates for novel industrial syntheses. And with base metal catalysts, these reactions can be more cost-effective than reactions with PGM catalysts. For a more thorough discussion of the potential applications of DuPhos and BPE ligands, check out our Snapshot on the topic (pdf).
Base metal catalysts may be a useful addition to your process and methodology development projects. From academic applications to large-scale projects, Sinocompound can work with you on custom synthesis projects to develop non-commercially available catalysts/ligands, including base metal complexes.
Learn more about Sinocompound’s catalyst products and services to accelerate your project to the next phase: https://en.sinocompound.com/Products_Services.html
1 a) M. J. Burk, J. Am. Chem. Soc. 1991, 113, 8518-8519; b) R. L. Harlow, W. A. Nugent, J. E. Feaster, M. J. Burk, , J. Am. Chem. Soc. 1993, 115, 10125-10138; c) W. A. Nugent, T. V. Rajanbabu, M. J. Burk, Science 1993, 259, 479-483
2 M. J. Burk, Acc. Chem. Res. 2000, 33, 363-372.
3 C. J. Cobley, I. C. Lennon, C. Praquin, A. Zanotti-Gerosa, Org. Proc. Res. Dev. 2003, 7, 407-411.
4 M. Shevlin, M. R. Friedfeld, H. Sheng, N. A. Pierson, J. M. Hoyt, L. C. Campeau, and P. J. Chirik, J. Am. Chem. Soc. 2016, 138, 3562-3569.
5 M. Shevlin, M. R. Friedfeld, H. Sheng, N. A. Pierson, J. M. Hoyt, L. C. Campeau, and P. J. Chirik, J. Am. Chem. Soc. 2016, 138, 3562-3569.
6 H. Zhong, M. Shevlin, P. J. Chirik, J. Am. Chem. Soc. 2020, 142, 5272-5281.
7 X. Du, Y. Xiao, J. M. Huang, Y. Zhang, Y. N. Duan, H. Wang, C. Shi, G. Q. Chen, X. Zhang, Nat. Commun., 2020, 11, 3239.
8 J. S. Zhou, X. Huang, S. Teng, and Y. R. Chi, Chem. Commun., 2021, 57, 3933-3936.
9 Y. Guo, M. C. Reis, J. Kootstra, and S. R. Harutyunyan, ACS Catal. 2021, 11, 8476-8483.