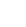
Blog Post: C-C cross-couplings featuring adamantyl-containing ligands
Release date:2022-11-24
Organometallic chemistry has been crucial to the evolution of cross-coupling reactions over the past 20 years, largely due to advances in ancillary ligand design. In this four-part blog series, we talk about the rise of bulky adamantyl-containing phosphine ligands and their suitability for industrial applications. In this third blog of our series, we explore the scope of adamantyl-containing ligands in pharma for C-C bond-forming reactions.
In our previous blog, we explained why the easy scale-up and desirable steric bulk of adamantyl-containing ligands made them promising candidates for industrial applications. In fact, many catalytic cross-coupling reactions featuring adamantyl-containing ligands are already used in pharmaceutical companies including Roche, Bayer, Merck and Pfizer.
Ad2PnBu-Pd-G-catalyzed Suzuki-Miyaura reactions
The Pd-catalyzed Suzuki-Miyaura cross-coupling reaction has been instrumental in industry over recent years. The importance of this reaction was formally recognized when Prof. Suzuki jointly received the Nobel Prize in chemistry in 2010 alongside Prof. Negishi and Prof. Heck for palladium-catalyzed cross couplings in organic synthesis.
Adamantyl-containing phosphine ligands are now entering the arena – particularly Ad2PnBu-Pd catalysts – helping pharmaceutical companies with their drug discovery
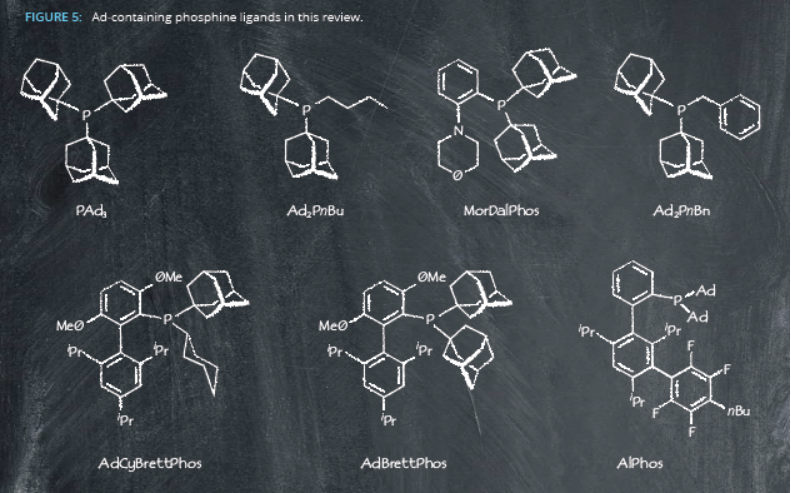
Roche was the first to introduce Ad2PnBu-Pd catalysts into their arsenal in 2018, in the synthesis of an antibacterial agent1. The G2-catalyst enabled formation of the core bi-pyridyl motif across a range of compounds. The G3-catalyst was later used by Bayer for developing cardiovascular disease treatments, forming the biphenyl block of substituted phenylalanine derivatives on a gram-
Ad2PnBu-Pd-G catalysts are suitably scalable, too: Pfizer were able to synthesize multigram quantities of their small molecule PCSK9 inhibitor5, and Merck reported a multigram synthesis of the cinnamate derivate intermediate for biaryl chroman agoPAMs6. This catalytic system is formed from Pd(OAc)2 in combination with Ad2PnBu, which was also successful for GSK and AstraZeneca in the synthesis of precursors to ATAD2 inhibitors in moderate yields7.
An even bigger challenge
One of the difficulties with cross-coupling reactions is chemoselectivity, particularly when selective mono-functionalization is desired. When cross-coupling reactions are performed, often a mixture of products is obtained, requiring further purification. In our previous blog, we mentioned the importance of catalyst and ligand screening for each type of reaction. Evaluation of different phosphine ligands can successfully improve mono-functionalization of pharmaceutically important compounds.
When optimizing a Suzuki-Miyaura cross-coupling to obtain unsymmetrical diarylpyridines, a selection of phosphine ligands was evaluated, including typical XPhos, SPhos, and RuPhos ligands. While these ligands gave reasonable product yields, switching to Ad2PnBn as the ligand gave 99% conversion in only 20 minutes8.
Another synthetically challenging reaction is the direct arylation of pyridines, as the highly Lewis basic sp2 nitrogen can interfere. However, using a Ad2PnBu-Pd-G3 catalyst in conjunction with a directing group strategy successfully achieved regioselective C3/C4 arylations9. The catalytic system enabled low-cost chloro- and bromoarenes to be used as coupling partners.
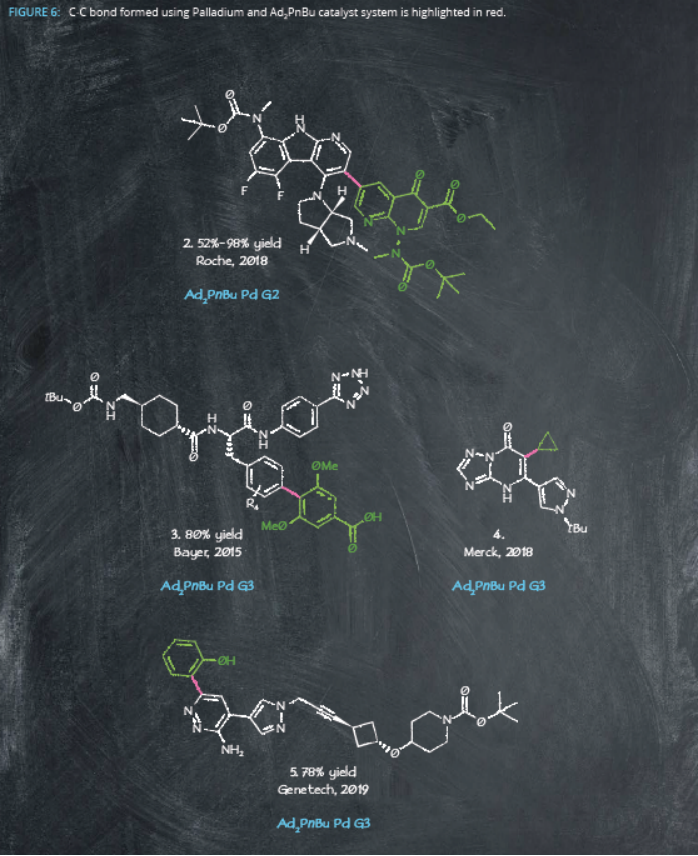
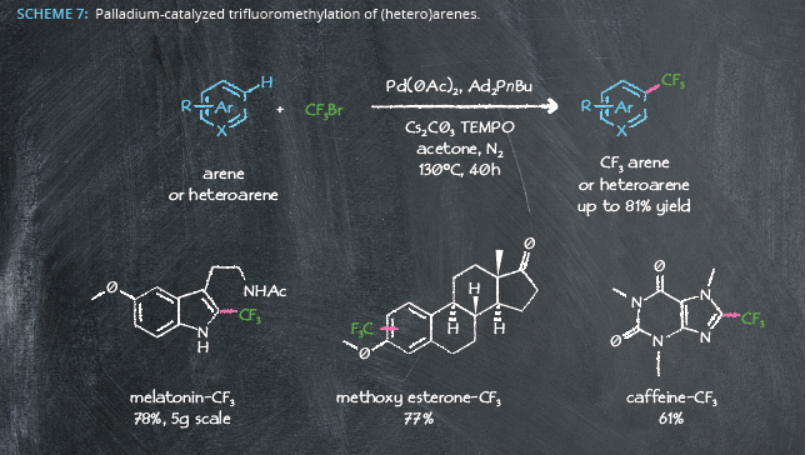
In fact, Ad2PnBu-Pd systems show further promise in another industrially important C-H functionalization reaction: trimethylfluorination. Around 20% of marketed drugs contain fluorine10, and the catalyst produced several trifluoromethyl-substituted (hetero)arenes under relatively mild reaction conditions. These reactions were effective at the multigram scale11, again highlighting the suitability of adamantyl-containing ligands for
The versatility of adamantyl-based ligands
Although Suzuki reactions and other related cross-coupling reactions are extensively used across industry, there are several other areas where adamantyl-based ligands are suitable. Recently, researchers have focused on developing copper-free Sonogashira cross-couplings (known as the Heck alkynylation reaction), but these are relatively challenging12.
Ad2PnBu-Pd-G3 catalysts show excellent promise here, as demonstrated by Novartis13. Under micellar aqueous reaction conditions, the catalytic system gave an increased yield of representative products compared to previously reported cBRIDP and dppf ligands. Additionally, the procedure worked when reducing catalyst loading from 3 to 1%, further improving commercialization prospects.
So, adamantyl-based phosphine ligands show great versatility across a range of industrially relevant C-C cross-coupling reactions and are promising for commercialization thanks to demonstrated multigram procedures in pharma. In the final blog of the series, we'll highlight industrially relevant cross-couplings beyond C-C bond formation reactions from catalysts featuring adamantyl-containing ligands, including C-N, C-heteroatom, and C-halogen bonds.
Download our white paper here to learn more about advanced adamantyl-containing phosphine ligands for challenging cross-coupling reactions.
1 Dey Fabian et al., WO20181788041
2 R. Ulrike et al., WO2015044163
3 U. F. Mansoor et al., WO2018071283
4 A. P. Crew et al., US20190300521
5 A. Akin, M. T. Barrila, T. A. Brandt, A. M. R. Dechert-Schmitt, P. Dube, D. D. Ford, A. S. Kamlet, C. Limberakis, A. Pearsall, D. W. Piotrowski, B. Quinn, S. Rothstein, J. Salan, L. Wei, and J. Xiao, Org. Process Res. Dev. 2017, 21, 1990-2000.
6 C. W. Plummer, M. J. Clements, H. Chen et al., ACS Med. Chem. Lett. 2017, 8, 221-226.
7 P. Bamborough, C. Chung, E. H. Demont, R. C. Furze, A. J. Bannister, K. H. Che, H. Diallo, C. Douault, P. Grandi, T. Kouzarides, A. M. Michon, D. J. Mitchell, R. K. Prinjha, C. Rau, S. Robson, R. J. Sheppard, R. Upton, and R. J. Watson, Angew. Chem. Int. Ed. 2016, 55, 11382-11386.
8 Y. K. Jeon, J. Y. Lee, S. E. Kim, and W. S. Kim, J. Org. Chem. 2020, 85, 7399-7412
9 Org. Lett. 2016, 18, 6094-6097.
10 M. Inoue, Y. Sumii, N. Shibata, ACS Omega 2020, 10633-10640.
11 a) K. Natte, R. V. Jagadeesh, L. He, J. Rabeah, J. Chen, C. Taeschler, S. Ellinger, F. Zaragoza, H. Neumann, A. Brîckner, and M. Beller, Angew. Chem. Int. Ed. 2016, 55, 2782-2786; b) T. Christoph et al., WO2016071425
12 F. Mohajer, M.M. Heravi, V. Zadsirjan, N. Poormohammad, RSC Adv. 2021, 11, 6885-6925.
13 M. Jakobi, F. Gallou, C. Sparr, and M. Parmentier, Helv. Chim. Acta 2019, 102, e1900024