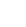
Blog Post: Adamantyl-containing ligands: applications beyond C-C bond forming reactions
Release date:2023-01-06
Organometallic chemistry has been crucial to the evolution of cross-coupling reactions over the past 20 years, largely due to advances in ancillary ligand design. In this four-part blog series, we talk about the rise of bulky adamantyl-containing phosphine ligands and their suitability for industrial applications. In previous blogs, we have dived into the challenges with respect to commercialization of this type of ligands, their high potential in industry applications and our most recent post on this topic highlighted the use of adamantyl-based ligands in C-C bond formation reactions. In this final blog, we explore the scope of adamantyl-containing ligands beyond C-C bond-forming reactions, and their future in industry.
Pd-catalysts featuring adamantyl-containing ligands have been applied in many industrially relevant C-C cross-coupling reactions, as we highlighted in our previous blog. However, the ligands have scope far beyond these reactions, and have been widely used for C-heteroatom bond formation reactions too. Here, we illustrate just how versatile adamantyl-containing ligands can be in Pd-catalysis.
Accessing C-N bond coupling with MorDalPhos
Ad2PnBu has so far been the dominant ligand for C-C reactions. However, C-heteroatom bond formation is an even trickier class of reactions, and it’s only in the past decade or so that adamantyl-based ligands have been effective here.
One of the prominent ligands in this area, MorDalPhos, is exceedingly effective in C-N bond formation. Developed in 2010 by Stradiotto1, it is highly active in chemoselective arylations of amines.
Since then, MorDalPhos has shown its ability in monoarylation reactions2, including hydrazine monoarylations3. The catalysts are scalable, which allowed the team to develop a library of 21 edaravone derivatives with a range of N-aryl substitutions. In fact, the first gram-scale N-arylation of amines using aryl phosphates was achieved with this ligand4, tolerating many common functional groups.
This adamantyl-based ligand is promising for industry too. When combined with [Pd(cinnamyl)Cl]2, it could synthesize a range of MALT 1 inhibitors5 and compounds for inflammatory disorders6. Merck also used the catalyst for forming C-N bonds between alkyl amines and aryl halides, synthesizing compounds for treating central nervous system disorders7 and bacterial infections8.
How scalable is Pd-catalyzed C-N cross-coupling?
While adamantyl-based ligands can be scaled up for industry, C-N cross-coupling itself can be trickier to scale up. Amines have low intrinsic acidity, which means that they generally require strong inorganic (or insoluble) bases to facilitate the reaction. The subsequent heterogeneous nature of the reaction can cause problems when scaled up, and so researchers are seeking out alternative reaction conditions.
Buchwald and team recently showed that an AlPhos-supported palladium catalyst is effective for the amination reaction using DBU as a base9. The team found adamantyl-based ligands were superior to other commonly used Buchwald ligands, giving almost quantitative yield of the cross-coupling products even at room temperature. Being able to use soluble organic bases such as DBU offer a more attractive solution for scaling up in industry.
Carbon couplings with heteroatoms and halides
We’ve established that adamantyl-ligands are effective in both C-C and C-N cross couplings. But did you know they’re effective for other bond formation reactions too?
C-O bond formation is extremely challenging, and there are a few instances where Pd-based catalysts can achieve this. For example, tBuBrettPhos is an effective ligand in this system, but doesn’t work as well when used with electron-rich aryl halide substrates10. However, switching to an AdCyBrettPhos ligand improved the yield in these traditionally difficult reactions11.
Adamantyl-based ligands can effectively promote C-halogen bond formation, too. Despite being a useful transformation for pharmaceutical and agrochemical industries, it’s still challenging to find general reaction conditions. AlPhos made a promising start, as a ligand for Pd-catalyzed fluorination of activated (hetero)aryl triflates12 - even in instances where regioselectivity could be an issue.
Efficient ligands for challenging reactions
Over this blog series, we’ve established that adamantyl-containing phosphine ligands are highly effective in many C-C and C-X bond forming reactions. Research groups continue to find new applications of adamantyl-phosphine based catalysts. Yet, despite their suitability for scale-up and versatility, there are still few large-scale applications reported. So, why is this?
In some instances, there may have been issues of ligand IP protection, limiting the use of adamantyl-based ligands. Additionally, many perceive this type of ligand as being difficult to synthesize. However, the value and potential impact of these ligands are being more widely understood, and large-scale applications in production will likely follow suit.
There are ways to facilitate the wider adoption of adamantyl-based ligands in industry. The main factor here is communication: academia, industrial research groups and catalyst and ligand manufacturers need to closely collaborate. Investing time into ligand development can overcome the synthetic challenges of adamantyl-based ligands, unlocking their potential in process development R&D and production, and beyond.
Download our white paper here to learn more about advanced adamantyl-containing phosphine ligands for challenging cross-coupling reactions.
1 R. J. Lundgren, B. D. Peters, P. G. Alsabeh, M. Stradiotto, Angew. Chem. Int. Ed. 2010, 49, 4071-4074.
2 M. Stradiotto, Chimica Oggi/Chemistry Today, 2012, 30, 64-67.
3 M. A. MacLean, E. Diez-Cecilia, C. B. Lavery, M. A. Reed, Y. Wang, D. F. Weaver, M. Stradiotto, Bioorg. Med. Chem. Lett. 2016, 26, 100-104.
4 Z. Chen, X. Chen, and C. M. So, J. Org. Chem. 2019, 84, 10, 6366-6376.
5 T. Lu et al., US20190381019
6 S. E. Van der Plas et al., WO2019076716
7 G. J. Morriello et al., WO2017000277
8 W. Liu et al., WO2017155765
9 J. M. Dennis, N. A. White, R. Y. Liu, and S. L. Buchwald, J. Am. Chem. Soc. 2018, 140, 4721-4725.
10 a) R. Szpera, P. G. Isenegger, M. Ghosez, N. J. W. Straathof, R. Cookson, D. C. Blakemore, P. Richardson, and V. Gouverneur, Org. Lett. 2020, 22, 16, 6573-6577; b) Z. Q. Song and D. H. Wang, Org. Lett. 2020, 22, 21, 8470-8474; c) Y. Otsuka, T. Yamamoto, K. Fukase, Chem. Asian J. 2019, 14, 2719-2723.
11 H. Zhang, P. R. Castillo, and S. L. Buchwald, Org. Lett. 2018, 20, 1580-1583.
12 A. C. Sather, H. G. Lee, V. Y. De La Rosa, Y. Yang, P. Müller, and S. L. Buchwald, J. Am. Chem. Soc. 2015, 137, 13433-13438.